Article
Phase stability of Li-Mn-O oxides as cathode materials for Li-ion Batteries: insights from ab-initio calculations
Physical Chemistry Chemical Physics
(2014)
Abstract
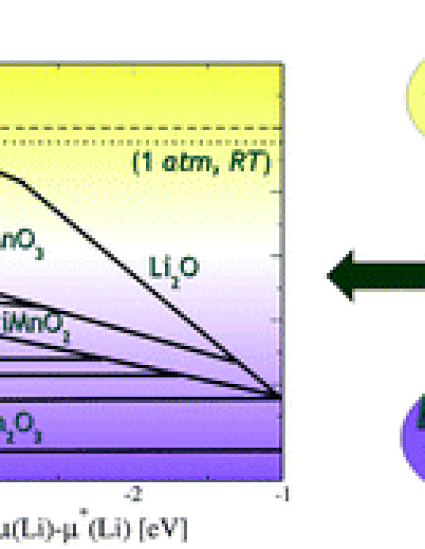
In this work, we present a density-functional theory (DFT) investigation of the phase stability, electrochemical stability and phase transformation mechanisms of the layered and over-lithiated Mn oxides. This study includes the thermodynamic stability of Li and oxygen vacancies, to examine the electrochemical activation mechanisms of these cathode materials. The DFT calculations provide phase diagrams of the Li–Mn–O system in both physical and chemical potential spaces, including the crystals containing vacancies as independent phases. The results show the ranges of electrochemical activity for both layered LiMnO2 and over-lithiated Li2MnO3. By using a thermodynamic model analysis, we found that the required temperature for oxygen evolution and Li vacancy formation is too high to be compatible with any practical synthesis temperature. Using solid-state transition calculations, we have identified the key steps in the phase transition mechanism of the layered LiMnO2 into the spinel phase. The calculated effects of pH on the Li–Mn–O phase stability elucidated the mechanism of Mn2+ formation from the spinel phase under acidic conditions.
Disciplines
Publication Date
April 8, 2014
DOI
10.1039/C4CP00937A
Citation Information
Roberto C. Longo, F. T. Kong, Santosh KC, M. S. Park, et al.. "Phase stability of Li-Mn-O oxides as cathode materials for Li-ion Batteries: insights from ab-initio calculations" Physical Chemistry Chemical Physics Vol. 16 Iss. 23 (2014) p. 11233 - 11242 ISSN: 1463-9076 Available at: http://works.bepress.com/santosh-kc/32/