Initial Three-Year Review of Transcatheter Aortic Valve Replacement (TAVR) Program Launch at a Tertiary Academic Community Hospital
Tara Stansbury, Research Scholar
Raymond Singer, MD Lehigh Valley Physicians Group - Cardiac Thoracic Surgery
Abstract
This study reviews the outcomes of the 225 Transcatheter Aortic Valve Replacement Procedures that were performed over the past three years at Lehigh Valley Health Network. It was performed as a retrospective chart review to evaluate the mortality and stroke rates for patient demographics such as age, gender, and various comorbidities. Patients performed better with decreasing comorbidities and a low percentage required pacemaker implantation after surgery. This study shows that TAVR procedures can be performed safely at a tertiary academic community hospital with excellent outcomes compared to the PARTNER trials and commercial roll-out studies.
Introduction
Severe Aortic Stenosis (AS) is the narrowing or obstruction of the aortic valve that results in fatigue, shortness of breath, and, in extreme cases, hospitalization or death.1 In the past, surgical aortic valve replacement (SAVR), which requires a sternotomy and cardiopulmonary bypass, was the only option for patients with this condition. During this procedure, the cardiothoracic surgeon opens the chest, removes the aortic valve and replaces it with a prosthetic valve.2 Many patients with aortic stenosis, however, are considered inoperable or high-risk due to age, frailty, and other comorbidities. These patients, often denied surgical aortic valve replacement (SAVR), are alternatively being considered for the less invasive transcatheter approach.3
Transcatheter aortic valve replacement (TAVR) is a transformative procedure that avoids the need for conventional open-heart aortic valve replacement in high-risk patients.3 These patients now have the option of TAVR, a less invasive procedure that uses a sheath to implant the prosthetic valve into the existing aortic valve. The catheter is fed into either the groin (transfemoral) or the chest (transapical) and then guided through the blood vessel into the aorta.4 The transfemoral approach is more common, however, the transapical approach is performed if the patient has coronary artery disease.2 Although the procedure is considered less invasive, it has not yet been cleared for low-risk patients. In order to be considered high-risk, the patient must have several comorbities that prevent him or her for being a viable candidate for surgical aortic valve replacement.4
The Food and Drug Administration approved TAVR in 20115 and shortly after on May 16, 2012, the team of cardiologists and cardiothoracic surgeons at Lehigh Valley Health Network (LVHN) started performing this innovative procedure.6 Three years later, 225 procedures have been performed with great success. The purpose of this analysis was to assess the outcomes of all the TAVR procedures conducted at LVHN, a tertiary academic community hospital, over the past three years. Mortality rates and stroke rates were evaluated based on certain patient demographics such as age, sex and relevant comorbidities. A study was also conducted to determine the rate of pacemaker implantation.
Methodology
A retrospective chart review was performed with approval of the Lehigh Valley Health Network Department of Surgery as a quality improvement (QI)/process improvement (PI) project with permission to report in the literature using the SQUIRE guidelines. The TAVR procedure was performed 225 times over the course of three years in patients with severe aortic stenosis. These patients were deemed inoperable, or high-risk, for surgical aortic valve replacement during the TAVR clinic and were therefore considered to be good candidates for the TAVR procedure. Age, gender, comorbidities, patient mortality, stroke, and pacemaker implantation were determined by reviewing the patient records. All patients were evaluated to determine the mortality and stroke rates. Only patients without pacemakers prior to surgery were evaluated to determine the rate of pacemaker implantation after surgery. The comparison data from the PARTNER Trials was taken from the study, “Clinical Outcomes at 1 Year Following Transcather Aortic Valve Replacement.”4
Results
A review of the first 3 years shows 225 TAVR procedures were performed, 134 Trans-Femoral (TF), 91 Trans-Apical (TA). The patient population was 120 men and 105 women, age ranging from 50 to 99, 41 of whom were less than 75 years old, 96 were 75 to 84 years old, 81 were 85 to 94 years old, and 1 was greater than 95 years old. Overall in-hospital mortality was 4 (1.78%); 30-day mortality 7 (3.11%); 60-day mortality 8 (3.56%); 1-year mortality 27 (12%). Complications included stroke 11 (4.89%), need for pacemaker 41 (21.81%). The 4 in-hospital deaths were due to stroke (1), liver failure (1), cardiac failure (1), and hemorrhage (1). Regrettably, some of the patient demographics only contained a few individuals for LVHN in comparison to the many STS/AVT Registry patients. Otherwise, the LVHN results compared favorably to the PARTNER trials and other commercial roll-out studies.
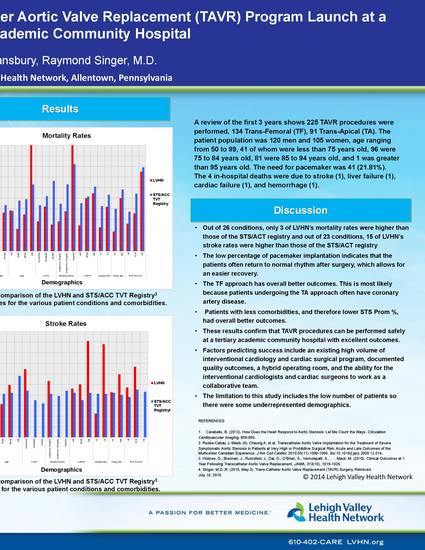
Figure 1: A comparison of the LVHN and STS/ACC TVT Registry4 Mortality rates for the various patient conditions and comorbidities.
Figure 2: A comparison of the LVHN and STS/ACC TVT Registry4 Stroke rates for the various patient conditions and comorbidities.
Discussion
The purpose of this study was to assess the outcomes of all the TAVR procedures that were performed over the past three years. Out of 26 conditions, only 3 of LVHN’s mortality rates were higher than those of the STS/ACT registry and out of 23 conditions, 15 of LVHN’s stroke rates were higher than those of the STS/ACT registry. The low percentage of pacemaker implantation indicates that the patients often return to normal rhythm after surgery, which allows for an easier recovery. Pacemaker implantation requires an additional procedure that requires a longer stay for the patient and additional bills that are unfavorable for both the hospital and the patient. In general, patients performed better with lower age, higher left ventricular ejection fraction (LVEF%), zero to moderate COPD, lower creatinine levels, and no preoperative atrial fibrillation. The transfemoral approach has overall better outcomes. This is most likely because patients undergoing the transapical approach often have coronary artery disease. Patients with less comorbidities, and therefore lower STS Prom %, had overall better outcomes.
Altogether, these results confirm that TAVR procedures can be performed safely at a tertiary academic community hospital with excellent outcomes compared to the PARTNER trials and commercial roll-out studies. Factors predicting success include an existing high volume of interventional cardiology and cardiac surgical program, documented quality outcomes, a hybrid operating room, and the ability for the interventional cardiologists and cardiac surgeons to work as a collaborative team.
The limitation to this study includes the low number of patients (225) at LVHN in comparison to the high number of patients in the PARTNER trials (12,182). Some of the demographics were underrepresented (e.g. only 1 patient over 95 years old) so, some of the statistics may be considered invalid. Another limiting factor is that not all patients receive the same brand of valve. While most of the patients received the Edwards SAPIEN valves, some patients received the Medtronic CoreValve. It is difficult to determine whether patients performed superior with one valve or the other because so few patients received the Medtronic CoreValve. For future work, it would be more beneficial to determine the mortality and stroke rates with a larger patient population and compare the different demographics between the two types of valves.
References:
- Carabello, B. (2013). How Does the Heart Respond to Aortic Stenosis: Let Me Count the Ways. Circulation:
Cardiovascular Imaging, 858-860.
- Walther, T., Simon, P., Dewey, T., Wimmer-Greinecker, G., Falk, V., Kasimir, M., . . . Mack, M. (2007).
Transapical Minimally Invasive Aortic Valve Implantation: Multicenter Experience. Circulation.
3. Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter Aortic Valve Implantation for the Treatment of Severe
Symptomatic Aortic Stenosis in Patients at Very High or Prohibitive Surgical Risk: Acute and Late
Outcomes of the Multicenter Canadian Experience. J Am Coll Cardiol. 2010;55(11):1080-1090.
doi:10.1016/j.jacc.2009.12.014.
4. Holmes, D., Brennan, J., Rumsfeld, J., Dai, D., O’Brien, S., Vemulapalli, S., . . . Mack, M. (2015). Clinical
Outcomes at 1 Year Following Transcatheter Aortic Valve Replacement. JAMA, 313(10), 1019-1026.
5. U.S. Food and Drug Administration. (2013, September 6) Retrieved July 9, 2014, from
6. Singer, M.D.,R. (2015, May 3). Trans-Catheter Aortic Valve Replacement (TAVR) Surgery, Retrieved
July 16, 2015.
Stansbury, T.; Singer, R., (2015, July 31) Initial Three-Year Review of Transcatheter Aortic Valve Replacement (TAVR) Program Launch at a Tertiary Academic Community Hospital. Poster presented at LVHN Research Scholar Program Poster Session, Lehigh Valley Health Network, Allentown, PA.

Copyright © Lehigh Valley Health Network