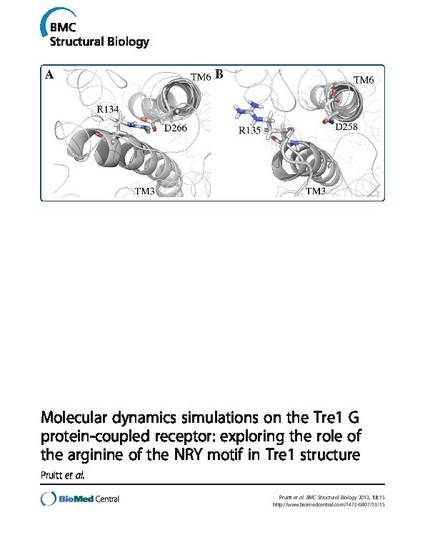
Abstract Background: The arginine of the D/E/NRY motif in Rhodopsin family G protein-coupled receptors (GPCRs) is conserved in 96% of these proteins. In some GPCRs, this arginine in transmembrane 3 can form a salt bridge with an aspartic acid or glutamic acid in transmembrane 6. The Drosophila melanogaster GPCR Trapped in endoderm-1 (Tre1) is required for normal primordial germ cell migration. In a mutant form of the protein, Tre1sctt, eight amino acids RYILIACH are missing, resulting in a severe disruption of primordial germ cell development. The impact of the loss of these amino acids on Tre1 structure is unknown. Since the missing amino acids in Tre1sctt include the arginine that is part of the D/E/NRY motif in Tre1, molecular dynamics simulations were performed to explore the hypothesis that these amino acids are involved in salt bridge formation and help maintain Tre1 structure. Results: Structural predictions of wild type Tre1 (Tre1+) and Tre1sctt were subjected to over 250 ns of molecular dynamics simulations. The ability of the model systems to form a salt bridge between the arginine of the D/E/NRY motif and an aspartic acid residue in transmembrane 6 was analyzed. The results indicate that a stable salt bridge can form in the Tre1+ systems and a weak salt bridge or no salt bridge, using an alternative arginine, is likely in the Tre1sctt systems. Conclusions: The weak salt bridge or lack of a salt bridge in the Tre1sctt systems could be one possible explanation for the disrupted function of Tre1sctt in primordial germ cell migration. These results provide a framework for studying the importance of the arginine of the D/E/NRY motif in the structure and function of other GPCRs that are involved in cell migration, such as CXCR4 in the mouse, zebrafish, and chicken.
Available at: http://works.bepress.com/monica_lamm/2/

This article is from BMC Structural Biology, 13 (2013): article no. 15, doi: 10.1186/1472-6807-13-15.