Article
Regioselective cross metathesis for block and heterotelechelic polymer synthesis
Polymer Chemistry
(2016)
Abstract
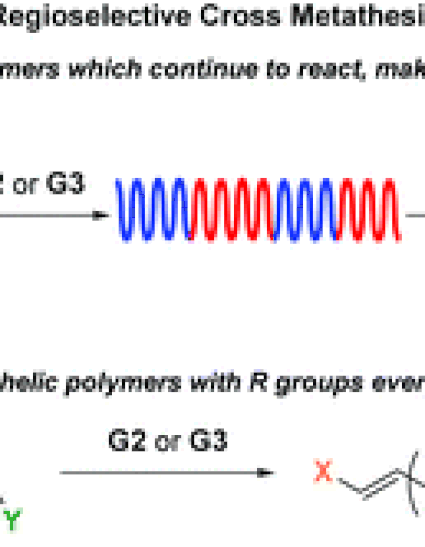
Ring-opening metathesis polymerization (ROMP) of 3-substituted cyclooctene (3RCOE) monomers with Grubbs 2nd and 3rd generation catalysts (G2 and G3) has been shown to occur with high head-to-tail regioregularity and E-stereoregularity. Cross metathesis (CM) between the resultant polymer chains, a secondary metathesis reaction during ROMP, also results in head-to-tail regioregularity and E-stereoregularity. The selectivity of the CM is demonstrated in the reaction between polymers of various molar masses or with different R substituents. In the latter case, monitoring by DSC reveals the change from a multiblock polymer to a statistical copolymer by displaying first two, then one Tg. The regioregularity of both the ROMP and CM reactions is exploited for the synthesis of heterotelechelic polyolefins through the use of an asymmetric, allylically-substituted chain transfer agent alongside the 3RCOE monomer.
Disciplines
Publication Date
2016
DOI
10.1039/C6PY01231K
Citation Information
Madalyn R. Radlauer, Megan E. Matta and Marc A. Hillmyer. "Regioselective cross metathesis for block and heterotelechelic polymer synthesis" Polymer Chemistry Iss. 40 (2016) p. 6269 - 6278 ISSN: 1759-9954 Available at: http://works.bepress.com/madalyn-radlauer/5/
