Article
Protein cross-linking capillary electrophoresis at increased throughput for a range of protein–protein interactions
Analyst
(2018)
Abstract
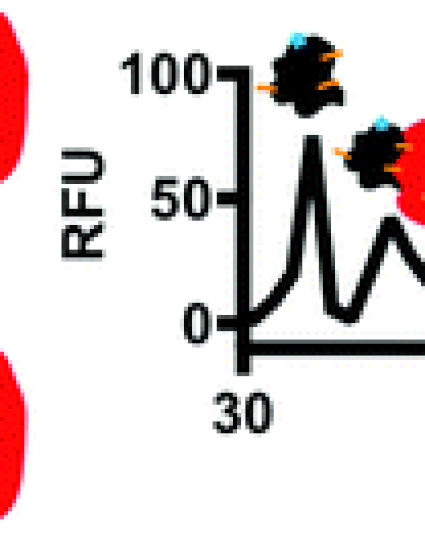
Tools for measuring affinities and stoichiometries of protein–protein complexes are valuable for elucidating the role of protein–protein interactions (PPIs) in governing cell functions and screening for PPI modulators. Such measurements can be challenging because PPIs can span a wide range of affinities and include stoichiometries from dimers to high order oligomers. Also, most techniques require large amounts of protein which can hamper research for difficult to obtain proteins. Protein cross-linking capillary electrophoresis (PXCE) has the potential to directly measure PPIs and even resolve multiple PPIs while consuming attomole quantities. Previously PXCE has only been used for high affinity, 1 : 1 complexes; here we expand the utility of PXCE to access a wide range of PPIs including weak and multimeric oligomers. Use of glutaraldehyde as the cross-linking agent was key to advancing the method because of its rapid reaction kinetics. A 10 s reaction time was found to be sufficient for cross-linking and quantification of seven different PPIs with Kd values ranging from low μM to low nM including heat shock protein 70 (Hsp70) interacting with heat shock organizing protein (3.8 ± 0.7 μM) and bcl2 associated anthanogene (26 ± 6 nM). Non-specific cross-linking of protein aggregates was found to be minimal at protein concentrations <20 μM as assessed by size exclusion chromatography. PXCE was sensitive enough to measure changes in PPI affinity induced by the protein nucleotide state or point mutations in the protein-binding site. Further, several interactions could be resolved in a single run, including Hsp70 monomer, homodimer and Hsp70 complexed the with c-terminus of Hsp70 interacting protein (CHIP). Finally, the throughput of PXCE was increased to 1 min per sample suggesting potential for utility in screening.
Disciplines
Publication Date
March 15, 2018
DOI
10.1039/C7AN02098H
Citation Information
Claire M. Ouimet, Mohamed Dawod, James Grinias, Victoria A. Assimon, et al.. "Protein cross-linking capillary electrophoresis at increased throughput for a range of protein–protein interactions" Analyst Vol. 143 Iss. 8 (2018) p. 1805 - 1812 ISSN: 1364-5528 Available at: http://works.bepress.com/james-grinias/9/
