Article
Total Synthesis of Spirastrellolide F Methyl Ester—Part 2: Macrocyclization and Completion of the Synthesis
Angewandte Chemie
(2009)
Abstract
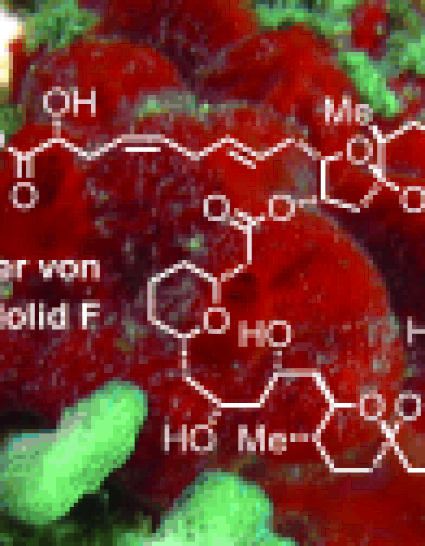
Wonder Lake: The compact and highly convergent total synthesis of the methyl ester of the marine macrolide spirastrellolide F was completed. The northern and southern "hemispheres" in only two stages were without intermediate protecting group manipulations combined (Suzuki coupling, Yamaguchi lactonization).
Keywords
- Marine macrolide spirastrellolide F
Disciplines
Publication Date
December, 2009
DOI
10.1002/ange.200906122
Publisher Statement
Copyright © 1999 - 2016 John Wiley & Sons, Inc. All Rights Reserved
Citation Information
Gregory W. O'Neil, Stefan Benson, Marie-Pierre Collin, Julien Ceccon, et al.. "Total Synthesis of Spirastrellolide F Methyl Ester—Part 2: Macrocyclization and Completion of the Synthesis" Angewandte Chemie Vol. 121 Iss. 52 (2009) p. 10130 - 10134 Available at: http://works.bepress.com/gregory_oneil/18/
