Article
Divergent pathways to isophthalates and naphthalate esters from methyl coumalate
Tetrahedron Letters
Document Type
Article
Disciplines
Publication Version
Accepted Manuscript
Publication Date
11-7-2018
DOI
10.1016/j.tetlet.2018.09.058
Abstract
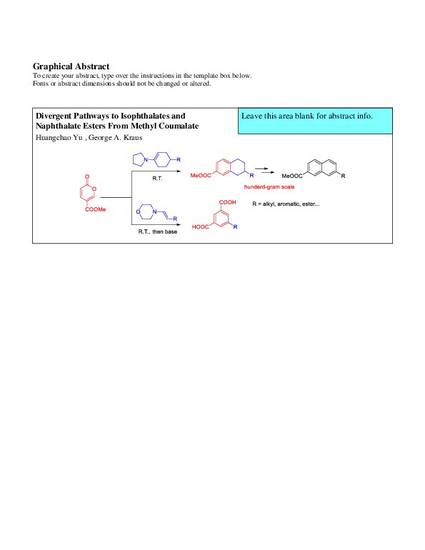
Methyl coumalate readily reacts with enamines at ambient temperature to give lactones, which can be further transformed into isophthalates and tetrahydronaphthoates. Both cyclic and acyclic enamines show good reactivity. Dehydrogenation of tetrahydronaphthoate 4a was achieved on a hundred-gram scale.
Creative Commons License
Creative Commons Attribution-NonCommercial-No Derivative Works 4.0 International
Copyright Owner
Elsevier Ltd.
Copyright Date
2018
Language
en
File Format
application/pdf
Citation Information
Huangchao Yu and George A. Kraus. "Divergent pathways to isophthalates and naphthalate esters from methyl coumalate" Tetrahedron Letters Vol. 59 Iss. 45 (2018) p. 4008 - 4010 Available at: http://works.bepress.com/george_kraus/256/

This is a manuscript of an article published as Yu, Huangchao, and George A. Kraus. "Divergent pathways to isophthalates and naphthalate esters from methyl coumalate." Tetrahedron Letters 59, no. 45 (2018): 4008-4010. DOI: 10.1016/j.tetlet.2018.09.058. Posted with permission.