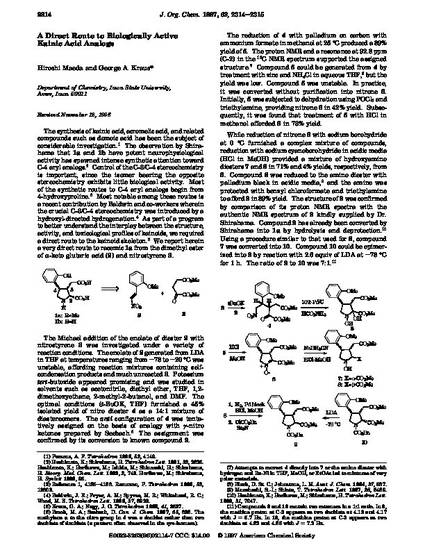
The synthesis of kainic acid, acromelic acid, and related compounds such as domoic acid has been the subject of considerable investigation.1 The observation by Shirahama that 1a and 1b have potent neurophysiological activity has spawned intense synthetic attention toward C-4 aryl analogs.2 Control of the C-3/C-4 stereochemistry is important, since the isomer bearing the opposite stereochemistry exhibits little biological activity. Most of the synthetic routes to C-4 aryl analogs begin from 4-hydroxyproline.3 Most notable among these routes is a recent contribution by Baldwin and co-workers wherein the crucial C-3/C-4 stereochemistry was introduced by a hydroxyl-directed hydrogenation.4 As part of a program to better understand the interplay between the structure, activity, and toxicological profiles of kainoids, we required a direct route to the kainoid skeleton.5 We report herein a very direct route to racemic 1a from the dimethyl ester of R-keto glutaric acid (2) and nitrostyrene 3.
Available at: http://works.bepress.com/george_kraus/20/

Reprinted (adapted) with permission from The Journal of Organic Chemistry, 62(8); 2314-2315. Doi: 10.1021/jo962114s. Copyright 1997 American Chemical Society.