Article
Rate coefficient determinations for H+NO2 → OH+NO from high pressure flow reactor measurements
The Journal of Physical Chemistry A
(2015)
Abstract
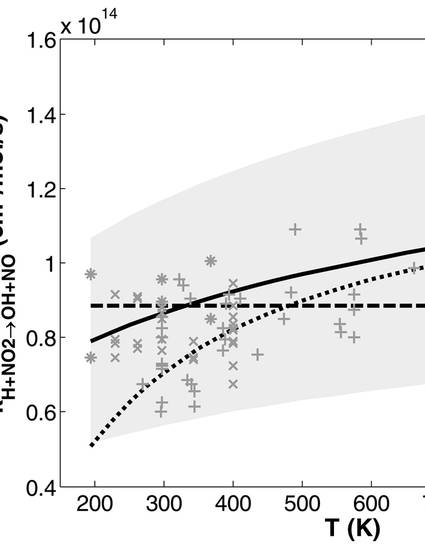
Rate coefficients for the reaction H + NO2 → OH + NO (R1) have been determined over the nominal temperature and pressure ranges of 737–882 K and 10–20 atm, respectively, from measurements in two different flow reactor facilities: one laminar and one turbulent. Considering the existing database of experimental k1 measurements, the present conditions add measurements of k1 at previously unconsidered temperatures between ∼820–880 K, as well as at pressures that exceed existing measurements by over an order of magnitude. Experimental measurements of NOx-perturbed H2 oxidation have been interpreted by a quasi-steady state NOx plateau (QSSP) method. At the QSSP conditions considered here, overall reactivity is sensitive only to the rates of R1 and H + O2 + M → HO2 + M (R2.M). Consequently, the ratio of k1 to k2.M may be extracted as a simple algebraic function of measured NO2, O2, and total gas concentrations with only minimal complication (within measurement uncertainty) due to treatment of overall gas composition M that differs slightly from pure bath gas B. Absolute values of k1 have been determined with reference to the relatively well-known, pressure-dependent rate coefficients of R2.B for B = Ar and N2. Rate coefficients for the title reaction determined from present experimental interpretation of both laminar and turbulent flow reactor results appear to be in very good agreement around a representative value of 1.05 × 1014 cm3 mol–1 s–1 (1.74 × 10–10 cm3 molecule–1 s–1). Further, the results of this study agree both with existing low pressure flash photolysis k1 determinations of Ko and Fontijn (J. Phys. Chem. 95 3984) near 760 K as well as a present fit to the theoretical expression of Su et al. (J. Phys. Chem. A 106 8261). These results indicate that, over the temperature range considered in this study and up to at least 20 atm, net chemistry due to stabilization of the H–NO2 reaction intermediate to form isomers of HNO2 may proceed at negligible rates compared to R1.
Disciplines
Publication Date
2015
DOI
10.1021/acs.jpca.5b01231
Citation Information
Francis M. Haas and Frederick L. Dryer. "Rate coefficient determinations for H+NO2 → OH+NO from high pressure flow reactor measurements" The Journal of Physical Chemistry A Vol. 119 Iss. 28 (2015) p. 7792 - 7801 Available at: http://works.bepress.com/francis-haas/9/
