Article
Modular origins of biological electron transfer chains
Proceedings of the National Academy of Sciences of the United States of America
(2018)
Abstract
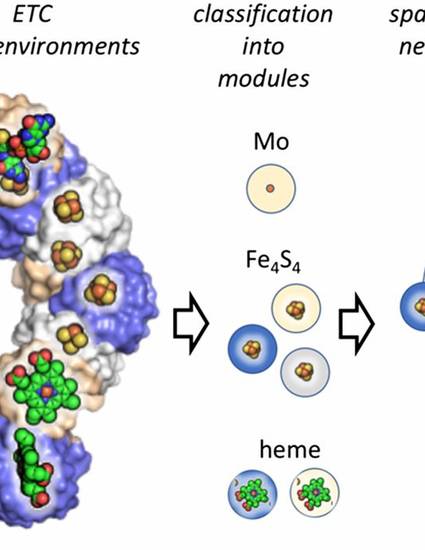
Oxidoreductases catalyze electron transfer reactions that ultimately provide the energy for life. A limited set of ancestral protein-metal modules are presumably the building blocks that evolved into this diverse protein family. However, the identity of these modules and their path to modern oxidoreductases is unknown. Using a comparative structural analysis approach, we identify a set of fundamental electron transfer modules that have evolved to form the extant oxidoreductases. Using transition metal-containing cofactors as fiducial markers, it is possible to cluster cofactor microenvironments into as few as four major modules: bacterial ferredoxin, cytochrome c, symerythrin, and plastocyanin-type folds. From structural alignments, it is challenging to ascertain whether modules evolved from a single common ancestor (homology) or arose by independent convergence on a limited set of structural forms (analogy). Additional insight into common origins is contained in the spatial adjacency network (SPAN), which is based on proximity of modules in oxidoreductases containing multiple cofactor electron transfer chains. Electron transfer chains within complex modern oxidoreductases likely evolved through repeated duplication and diversification of ancient modular units that arose in the Archean eon.
Disciplines
Publication Date
February 6, 2018
DOI
10.1073/pnas.1714225115
Citation Information
Hagai Raanan, Douglas H. Pike, Elisha Moore, Paul G. Falkowski, et al.. "Modular origins of biological electron transfer chains" Proceedings of the National Academy of Sciences of the United States of America Vol. 115 Iss. 6 (2018) p. 1280 - 1285 Available at: http://works.bepress.com/eli-moore/19/
