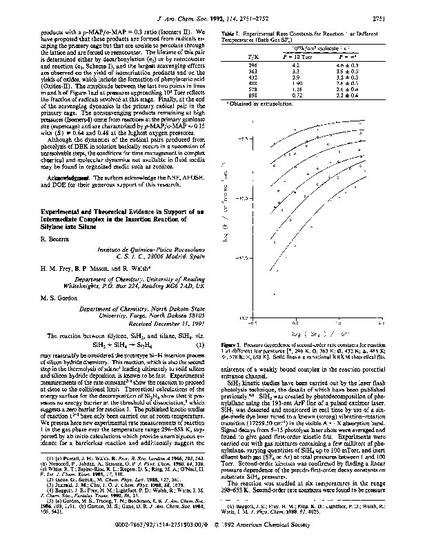
The reaction between silylene, SiH2, and silane, SiH4, viz. SiH2 + SiH4 - Si2H6 (1) may reasonably be considered the prototype Si-H insertion process of silicon hydride chemistry. This reaction, which is also the second step in the thermolysis of silane1 leading ultimately to solid silicon and silicon hydride deposition, is known to be fast. Experimental measurements of the rate constant2-4 show the reaction to proceed at close to the collisional limit. Theoretical calculations of the energy surface for the decomposition of Si2H6 show that it possesses no energy barrier at the threshold of dissociation,5 which suggests a zero barrier for reaction 1. The published kinetic studies of reaction 12"4 have only been carried out at room temperature. We present here new experimental rate measurements of reaction 1 in the gas phase over the temperature range 296-658 K, supported by ab initio calculations which provide unambiguous evidence for a barrierless reaction and additionally suggest the existence of a weakly bound complex in the reaction potential entrance channel.
Available at: http://works.bepress.com/mark_gordon/116/
